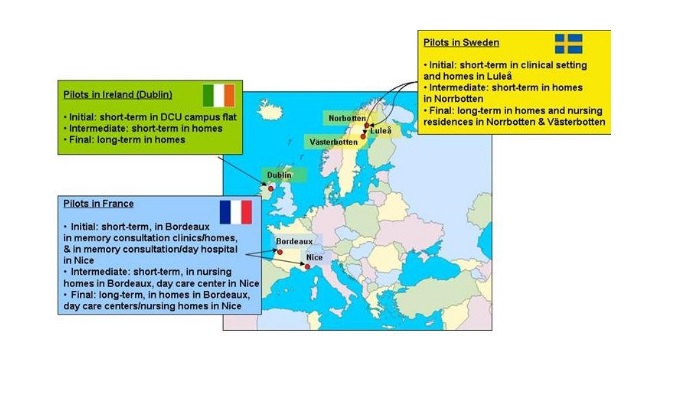
Pilots Overview
Evaluation and clinical validation of Dem@Care’s solution will be realized in three stages in synchronization with the three releases of the integrated systems prototypes. Pilots will take place in three countries: France, Sweden and Ireland and in three different environments:
Lab based: @Lab pilot The Lab-based pilot is going to be used as a reference site to test Dem@Care technologies and to acquire clinical knowledge about the behaviour of dementia patients and interaction with Information Communication Technologies (ICT). The acquired expertise will be used to drive deployment of ICT solutions in terms usability, functionality and reliability in the Nursing-Home and Home pilots. The three themes of enablement, diagnosis and safety permeate the three protocols. The labbased research is primarily concerned with diagnoses.
@ Lab pilot sites:
CHUN Hospital in Nice
Location
Observation Room in the CHUN Hospital in Nice, France. Resources and Research Memory Centre.
Participants
| DIAGNOSIS GROUP | INCLUSION AND NO INCLUSION CRITERIA |
| Normal Control group : n=50 | Inclusion criteria
Special cases: May be included: Subjects with no schooling aged from 50 to 79 years with MMSE> 22/30, and for over 80 years MMSE> 21/30 (standards of Kalafat, 2003) (Folstein et al. 1975), or arguments in favor of the following diagnosis: probable Alzheimer’s disease according to the criteria of the NINCDS-ADRDA and / or major depressive episode according to DSM-IV-R;
No inclusion Criteria
|
| Predementia
MCI Patients group : n =50
|
Inclusion criteria
No inclusion Criteria
|
| Dementia
AD patients group : n=50 |
Inclusion criteria
No inclusion Criteria
|
Specific Objectives
- Primary aim: To differentiate early stage Alzheimer’s disease from healthy control participants using actimeter and audio-video data analyses obtained during the completion of a standardized scenario of daily living oriented activities.
- Secondary aims: a). To ddifferentiate early stage Alzheimer’s disease or related disorder from patients with mild to moderate stages of the disease (b) to assess the impact of behavioral disturbances, in particular apathy, on the completion of the proposed activities of daily living, (c) to assess the impact of cognitive decline on speaking behavior and voice sound characteristics, (d) to assess the adjunct feasibility of the actigraphy coupled with an audio-video setting to a normal memory consultation, (e) to estimate the acceptability of this evaluation method by the participant during a standard consultation in a memory center, (f)to assess the participants’ acceptability to introduce a follow-up monitoring system based on the use of ICT within their own house.
Nursing Home based: @ Nursing Home
For the nursing home, functionality will be limited to the exercise, mood and sleep domains for Phase 1 since in this environment these are seen to be the prioritised domains. For this environment, personalisation is less fundamental than for the home-based deployment, since the enabling, person-centred approach is more appropriate for use in older adults with early stage dementia. For later stage dementia such as individuals in a nursing home, it is likely that more care decisions will lay in the hands of the clinician.
@ Nursing Home pilot sites:
Luleå University of Technology
Location
The researchers are based at the campus of Luleå University of Technology and the County Council of Norrbotten in Luleå, Sweden.
Participants & Duration
The participants are residents of Nursing homes and will be recruited consecutively through the time frame of the project and the three test periods. At the test of the first pilot device the number of participants will only be 4-5 people with dementia and the test will only be performed in Luleå for a time period of 3-4 days for each person. When the be Dem@Care device has matured and all important functionalities are in place more participants will be included in the tests both in Luleå, Sweden and in Nice, France. The participants should have a diagnosis of dementia, be residents of a nursing home, and have indication of Behavioral and Psychological symptoms of Dementia (BPSD). Over the whole time period of the project it is estimated that in all about 50 people with dementia will have tested the Dem@Care device for a time period of three months for each participant.
Specific Objectives
The @Nursing home evaluation will answer following questions:
- What is the usefulness, including acceptability and functionality, of the Dem@care technology in this context?
- Can the information from the Dem@care system support staff members’ reasoning when doing assessments and evaluations of intervention efficacies among people with BPSD?
- Can support of people with BPSD be more effective with the support of the Dem@care technology?
Collaboration with Nursing Home(s)
The @Nursing home evaluation will have collaboration with Geriatricians at both the Sunderby Hospital in Luleå and the CHU in Nice in the evaluation process.
Home based: @Home
The Home based pilot is primarily concerned with the theme of enablement. Within enablement, there are 5 functional domains, as defined in deliverable D2.2: Functional Requirements and Scenarios v1; Sleep, Exercise/Physical Activity, Activities of Daily Life(ADL/IADL) and Mood. These five areas will be investigated in the final versions of both the home and nursing home deployments.
@ Home pilot sites:
Dublin City University
Location
The researchers are based at the Dublin City University campus, in North County Dublin, Ireland. The participants are based in the surrounding Dublin area.
Participants
The @Home pilot in Dublin site will engage directly with “lead user” participants in order to facilitate a rolling iterative design process and expedite the final design of a workable prototype.. The Dem@Care @Home pilot will take place in the private homes of the lead users, who will be older adults with dementia, and their caregivers. Systems will be deployed to participants’ homes on a rolling basis until all participants are engaged with the Dem@Care system, for as long duration as the participants are happy to engage. All participants will be recruited via the Memory Works clinic at DCU.
Specific Objectives
The aims of the @Home pilot are to answer the following research questions;
- Is the system acceptable in the home, is it unintrusive and useful to the individual with dementia and their family caregivers?
- Are the functional requirements (as defined by clinicians in D2.2) reflective of the reported needs of the individual with dementia, as personally reported and reported by their family caregivers?
- What is the functional status of the individual as operationalised in the five functional areas described in D2.2 (Sleep, Exercise/Physical Activity, Activities of Daily Life(ADL/IADL) and Mood) and can the system optimise status in these areas?
- How autonomous and independent is the individual, and can deployment of the system support this autonomy?
Collaboration with Memory Clinic(s)
Collaboration will be with Memory Works Clinic at DCU and Alzheimer’s Cafe initiative but participants are not recruited through any hospital institution as they are not necessarily under the care of a hospital consultant or service.
The recruitment of people with dementia to participate in the pilot studies will be handled appropriately in Dem@Care, so as to ensure the fully informed consent of the participants, in line with European and National legislations
